
Static (12)
- Bimectin Injection; Anti-Parasitic; ivermectin; Injection; Cattle, Pigs;
- Bimectin Pour-on; Anti-Parasitic; ivermectin; Liquid; Cattle;
- Exodus; Anti-Parasitic; pyrantel pamoate; Oral Paste; Horses;
- Theraderm Cream; Anti-Microbial; triamcinolone acetonide; Cream; Dogs, Cats;
Accessibility Plan and Policies for Bimeda MTC Animal Health Inc.
This 2014-2021 accessibility plan outlines the policies and actions that Bimeda MTC Animal Health Inc. will put in place to improve opportunities for people with disabilities.
Our Commitment
Bimeda is committed to treating all people in a way that allows them to maintain their dignity and independence. We believe in integration and equal opportunity. We are committed to meeting the needs of people with disabilities in a timely manner and will do so by preventing and removing barriers to accessibility and meeting accessibility requirements under the Accessibility for Ontarians with Disabilities Act.
Workplace Emergency Response Information
Bimeda is committed to providing the customers and clients with publicly available emergency information in an accessible way upon request. We will also provide employees with disabilities with individualized emergency response information when necessary.
Bimeda will take the following steps to ensure employees are provided with emergency response information by January 1, 2015:
- Individualized workplace emergency response information will be provided to employees who have a disability, if the disability is such that the individualized information is necessary, and if Bimeda is aware of the need for accommodation due to the employee’s disability, Bimeda will provide this information as soon as is practicable after becoming aware of the need for accommodation
- Where the employee requires assistance, Bimeda will, with the consent of the employee, provide the workplace emergency response information to the person designated by Bimeda to provide assistance to the employee.
- Bimeda will review the individualized workplace emergency response information when the employee moves to a different location in the organization and when the employee’s overall accommodations needs or plans are reviewed. Completed and on-going.
Training
Bimeda will provide training to employees, volunteers and other staff members on Ontario’s accessibility laws and on the Human Rights Code as it relates to people with disabilities. Training will be provided in a way that best suits the duties of employees, volunteers and other staff members.
Bimeda will take the following steps to ensure employees are provided with the training needed to meet Ontario’s accessible laws by January 1, 2015:
- Develop and implement the necessary training materials.
- Ensure training is provided before or as soon as possible after commencement of employment.
- Keep and maintain records of training provided.
Completed
Information and Communications
Accessible Websites and Web Content
Bimeda is committed to meeting the communication needs of people with disabilities. We will consult with people with disabilities to determine their information and communication needs.
Bimeda’s current website is compliant to WCAG 2.0, Level A.
Bimeda will take the following steps to make all new websites and content on those sites conform to WCAG 2.0, Level AA by January 1, 2021:
- A review is underway of the required changes that need to be made to the website by January 1, 2021.
Completed
Feedback
Bimeda is committed to conducting a review of all feedback processes across the organization, both internally and externally, to ensure that processes are accessible to persons with disabilities by providing or arranging for accessible formats and communications supports upon request by January 1, 2015.
Bimeda will take the following steps to ensure existing feedback processes are accessible to people with disabilities upon request by January 1, 2015:
- Any feedback is encouraged and directed to the HR Manager either by telephone, in person, in writing or by email.
The Bimeda Canada website includes information to contact the Human Resources Manager for a copy of the AODA policy and plan in an accessible format that is suitable to the individual making the request. The link can be found at the bottom of the website under AODA and under the contacts link.
Employment Standards
Bimeda is committed to fair and accessible employment practices to all its prospective and current employees. As a result, we are committed to working towards meeting the legislative requirements as it relates to preventing and removing barriers to accessibility in the workplace and providing everyone, including employees and representatives with disabilities access to the same opportunities.
Bimeda will ensure that all employment standards meet accessibility requirements by January 1, 2016. We are working towards taking the following steps in three major areas of employment standards: Recruitment, Accommodations for Staff and Performance Management, Career Development and Redeployment.
Recruitment
Human Resources at Bimeda is committed to notifying its staff members and the public about the availability of accommodation for applicants with disabilities in its recruitment process.
We will take the following steps to notify the public and staff that, when requested, Bimeda will accommodate people with disabilities during the recruitment and assessment processes and when people are hired:
- Review and modify existing recruitment, assessment and selection processes and procedures. Completed.
- Job postings will be amended informing applicants that the posting is available in an accessible format if required. Completed.
- Job postings will be amended informing applicants that if accommodation is required during the interview, it is available upon request (using the language below). Completed
- Pre screening questions on our career site will be amended informing applicants that if accommodation is required during the interview, it is available upon request. The language will be as follows:
“Bimeda is committed to providing a barrier-free workplace. Do you require accommodation during the selection/interview process of which we should be aware? ☐ Yes ☐ No
If yes, please describe required accommodation:” Completed.
- Human Resources will notify job applicants, when they are individually selected to participate further in an assessment or selection process that accommodations are available upon request in relation to the material or processes to be used. Completed.
- If a selected applicant requests an accommodation, Bimeda will consult with the applicant and provide, or arrange for the provision of, a suitable accommodation in a manner that takes into account the applicant’s accessibility needs due to disability. Completed.
- When making offers of employment, Bimeda will notify the successful applicant of its policies for accommodating employees with disabilities. Completed.
- Develop and provide training to colleagues responsible for recruitment, assessment, selection and on-boarding to ensure compliance with accommodation. Completed.
Accommodations for Employees
Bimeda will ensure that we are creating and following measures for any employee who requires accommodation as a result of a disability. Bimeda will take the following steps to develop and put in place a process for developing individual accommodation plans and return-to-work policies for employees that require accommodation as a result of a disability:
- Bimeda will continue to inform its employees of its policies (and any updates to those policies) used to support employees with disabilities, including policies on the provision of job accommodations that take into account staff member’s accessibility needs due to disability. This information will be provided to new employees as soon as practicable after commencing employment. Completed and on-going.
- Upon request of an employee or representative with a disability, Bimeda will consult with the employee to provide, or arrange for the provision of, accessible formats and communication supports for information that is needed to perform his/her job, and information that is generally available to other employees, including information as it relates to conducting performance management, providing career development and advancement to employees, or when redeploying employees. Completed and on-going.
- In determining the suitability of an accessible format or communication support, Bimeda will consult with the employee making the request.
- Make employees aware of Bimeda ’s duty to make a workplace accessible under AODA with the following language in a poster to be placed on the Health & Safety Board
“The Accessibility for Ontarians with Disabilities Act is legislation aimed at making workplaces and businesses more accessible for individuals living with a disability in Ontario. Bimeda is committed to removing barriers for our employees living with a disability.
If you have a disability that could benefit from accommodation the workplace, please contact Human Resources to fill out an accommodation request form. Completed.
Implement an “Accommodation Request Form”. Include in the employee training session. Completed.
Return to Work Process
Bimeda maintains documented return to work process for its employees who have been absent from work due to a disability and who require disability-related accommodations in order to return to work. The return to work process outlines the steps Bimeda will take to facilitate the return to work and will include documented individual accommodation plans as part of the process. This return to work process will not replace or override any other return to work process created by or under any other statue (ie: Workplace Safety Insurance Act, 1997).
Bimeda will take the following steps to develop and put in place a process for return to work process:
- Review and modify the Return to work policy and process. Completed.
- Develop and provide training to managers and other colleagues responsible for supporting the return to work process. Completed.
Performance Management, Career Development and Redeployment
We will take the following steps to ensure the accessibility needs of employees with disabilities needs are taken into account if Bimeda is using performance management, career development and redeployment processes:
- Review and modify existing performance management, career development and redeployment processes to ensure that accessibility needs of employees are met. Completed and on-going.
- Develop and provide training to managers and other colleagues who are involved in performance management, career development and redeployment processes to ensure compliance with processes. Completed and on-going.
For more information on this accessibility plan, please contact
Laura Incitti, Human Resources Manager at:
Accessible formats of this document are available free upon request from:
Laura Incitti
Human Resources Manager
Phone: 519 654-8017
Email:
Source: Ministry of Community and Social Services
Global Excellence in Animal Health
 Welcome to the Bimeda Canada website.
Welcome to the Bimeda Canada website.
Bimeda is a leading global manufacturer, marketer and distributor of animal health products and veterinary pharmaceuticals.
Located in Cambridge, Ontario, Bimeda Canada is the country’s leading manufacturer of sterile veterinary pharmaceuticals, and also manufactures a range of syringe-based pastes and gels. Bimeda Canada’s comprehensive manufacturing portfolio includes products for the swine, dairy, beef and equine sectors, which are sold in Canada and around the world. Bimeda Canada is also home to one of our eight research and development laboratories, which form part of Bimeda’s global innovation programme.
Through ongoing expansion and strategic acquisition, Bimeda has established markets in more than eighty countries worldwide and has R&D, manufacturing and distribution capabilities across Europe, North America, South America, Africa, Asia and Australasia. Bimeda employs over employees worldwide and has its global headquarters in Dublin, Ireland.
To learn more about life at Bimeda Canada, read our ‘Why Bimeda’ brochure.
To learn more about Bimeda’s global presence, why not read our corporate brochure.
 Safely return unwanted medications and medical sharps.nbsp; Why return? You can make a difference. Improper disposal of medications and medical sharps is potentially harmful to people, animals and the environment. HPSA is a not-for-profit organization responsible for the effective and safe collection and disposal of unused and expired consumer health products.
Safely return unwanted medications and medical sharps.nbsp; Why return? You can make a difference. Improper disposal of medications and medical sharps is potentially harmful to people, animals and the environment. HPSA is a not-for-profit organization responsible for the effective and safe collection and disposal of unused and expired consumer health products.
Visit the HPSA website to learn more.
Dear Customers, Distributors, Suppliers, and Friends,
As we all continue to adapt to the fast-changing COVID-19 situation, we would like to inform you of the measures which Bimeda has put in place to safeguard our colleagues and ensure continuity of supply to our customers.
Welfare of all Bimeda colleagues
The health, safety and well-being of our colleagues, their families, and the wider community is our highest priority. We have adopted practices in line with the recommendations of the World Health Organisation, the CDC local governments and local health authorities.
These practices, together with our internal policies will ensure that we maintain safe working environments for all our Bimeda colleagues in the face of this pandemic.
Manufacture and Supply of Product
All Bimeda’s manufacturing facilities are currently fully operational and we continue to release product to market. We are liaising with our suppliers and channel partners to ensure continuity of supply to our customers.
Bimeda’s ability to supply the market has not currently been impacted by the COVID-19 pandemic and we have no immediate concerns. However, this is a fluid situation, and we will ensure our customers are kept updated as this situation evolves. We would like to reassure you that we are doing everything within our power to ensure that our customers continue to be supplied by our products.
Customer Queries
For any queries, customers should continue to call or email your local sales representative or customer service team. Please note that all our Field and Technical representatives as well as our customer service teams are working remotely.
Our Commitment
Bimeda and our customers play a vital role in ensuring the ongoing health and productivity of animals which form a critical part of human food supply and we remain committed to maintaining ongoing product availability at this challenging and unprecedented time.
Thank You
We all, as members of the global community, are facing a challenging and difficult time. To our Customers, Distributors, Suppliers and Friends we thank you for your continued support and we wish you all continued health and safety.
Donal Tierney, Group CEO
24th March 2020
About Bimeda Canada
About Bimeda Canada
Bimeda Canada operations are located in Cambridge, Ontario. Our facilities have been approved to manufacture and distribute FDA, EU, and Canadian regulated products, and offer global logistics capabilities. Bimeda Canada is proud to employ over 190 employees.
Bimeda Canada Headquarters
Cambridge, Ontario
190+
employees
1 manufacturing
facility
We have dedicated customer service, commercial and technical teams that are focused on providing the best products and services possible, to meet our customers’ needs.
Our state-of-the art R&D laboratories contribute to our global R&D program, which is committed to anticipating future needs of the animal health sector.
Customer
Service
Commercial
R&D
Labs
Bimeda Canada Product Range
We offer a comprehensive range of veterinary pharmaceuticals and animal health products for livestock, equine and companion animals. These include nutritional, anti-parasitics, anti-microbials, anti-inflammatories and sedatives.
Learn more about our global locations
Read our corporate brochure to learn more about Bimeda’s global presence
Manufacturing and Distribution
Canada Manufacturing and Distribution

Globally, Bimeda has 9 manufacturing facilities in 8 countries. One of these is located in Cambridge, Ontario.
Canada - Manufactured Brands
Our manufacturing facility in Canada produces many well-known and trusted brands, which are used in markets around the world and which play a vital role in optimising the health, welfare and productivity of livestock, as well as promoting the health and wellbeing of horses. From anti-microbial injections, to equine pastes, anti-parasitic injections, injectable NSAIDs and vitamins, popular brands manufactured in Bimeda Canada play a role in optimising the health of animals in North America, Europe and Latin America.
Health & Safety Culture
Bimeda Health and Safety Culture
The health and safety of our employees, contractors and visitors to site is a daily priority in every Bimeda facility.
Our culture of continuous improvement means that Bimeda environmental health and safety management systems are regularly reviewed and optimised; taking into account both industry best practice and input from our Bimeda safety colleagues at our facilities around the world.

Bimeda strives to achieve a goal of:
- ZERO environmental incidents
- ZERO occupational illnesses
- ZERO work-related incidents
To achieve this, we commit to:
- Providing a safe working environment for all Bimeda staff and associates,
- Recognising, respecting and communicating relevant legislation and Bimeda standards to all,
- The reporting, management and continuous reduction of known and suspected risk activities and conditions,
- Supporting the EHS priorities and objectives in all Bimeda facilities globally, and ensuring that Bimeda EHS policy is adhered to by all staff and associates.
We are proud of our sincere and ongoing commitment to optimising health and safety at all Bimeda facilities.
Read the Bimeda Health and Safety Charter in full.
The Bimeda Story
Bimeda – Driving Excellence in Animal Health For 60 Years!
For 60 years, Bimeda colleagues have been driven by a passion for providing science-driven solutions to optimise the health and wellbeing of animals. If your next career move sees you join Bimeda, you’ll be part of a global network of over colleagues who benefit from 6 decades’ of expertise in animal health, and who are committed to working together to find ever-better ways to exceed the expectations of our customers, as we develop new ways to enhance animal health, welfare and productivity.
But how did we get here? Let’s take a walk down memory lane and look at how Bimeda’s unwavering commitment to animal health has seen us grow from a small Irish enterprise, to a leading global player in the animal health industry.
In The Beginning…..
 The original premises of Bimeda Chemicals Ltd in Phibsboro, DublinBimeda Chemicals Limited was founded on 22nd April 1960, when a group of Irish veterinary surgeons set up a company to supply Irish veterinarians with quality veterinary products. The original company motto was ‘For And By The Profession’. As the company founders were themselves veterinarians, they had unrivalled knowledge of the needs and wants of their customer base and were able to develop a highly sought product portfolio.
The original premises of Bimeda Chemicals Ltd in Phibsboro, DublinBimeda Chemicals Limited was founded on 22nd April 1960, when a group of Irish veterinary surgeons set up a company to supply Irish veterinarians with quality veterinary products. The original company motto was ‘For And By The Profession’. As the company founders were themselves veterinarians, they had unrivalled knowledge of the needs and wants of their customer base and were able to develop a highly sought product portfolio.
 1963 Advertisement from the Journal of The Veterinary Medical Association of IrelandFrom the original premises in Phibsboro, Dublin, Bimeda Chemicals Ltd manufactured a range of veterinary remedies and also distributed a variety of third party products. As the 1963 advertisement from the Journal of The Veterinary Medical Association of Ireland shows, within just a few years Bimeda had a broad and exciting portfolio of products.
1963 Advertisement from the Journal of The Veterinary Medical Association of IrelandFrom the original premises in Phibsboro, Dublin, Bimeda Chemicals Ltd manufactured a range of veterinary remedies and also distributed a variety of third party products. As the 1963 advertisement from the Journal of The Veterinary Medical Association of Ireland shows, within just a few years Bimeda had a broad and exciting portfolio of products.
Bimeda quickly gained a reputation for both quality and reliability, and successfully drove awareness of the company’s product range through regular, innovative advertising campaigns. Bimeda’s passion for marketing continues to this day and is supported by our global network of marketing colleagues.
1973 - The Bimeda Acquisition and Expansion into the UK
In 1973 Bimeda Chemicals Limited was acquired by the current Irish owners. The family already owned Osmond & Sons (Dublin) Limited and went on to acquire Constant Chemicals Ltd in 1975. These three companies were subsequently brought together in a new location in Tallaght, Dublin. The new Tallaght facility served for many years as a multi-purpose production and warehousing unit; producing injectables, intramammaries, oral solutions, topicals and hygiene products. By this time, Bimeda had also established itself in the UK market, with a distribution base in Liverpool and a reputation for high quality products.
Expansion into Africa
With Bimeda now firmly established in both Ireland and the UK, ambitions moved towards expansion. The export business became a key strategic focus, with the East African market becoming particularly important.
Despite the challenges of both travel and communication at the time, Bimeda thrived; achieving rapid success in East Africa which has been sustained over many decades. Today Bimeda’s AMEA (Africa, Middle East and Asia) business has a physical presence in Kenya, Zambia, Uganda, Tanzania and China, with an export division which serves the entire AMEA region.

The 1990s - Coming To North America
Throughout the late 1980s and early 1990s Bimeda continued to grow, with a strong network of distributor and contract manufacture partners helping Bimeda products to reach veterinarians and farmers all over Europe and Africa.
However, the company had ambitions beyond the European and AMEA regions and in 1991, Bimeda commenced distributing products in the USA. In 1997, an acquisition followed, giving Bimeda its first permanent base in North America. A period of rapid expansion followed, and between 1997 and 2008, Bimeda undertook 5 key acquisitions in the USA, Canada and Mexico, creating a strong R&D, manufacturing and commercial presence throughout the North American continent.
A Decade of Global Acquisitions
2010 marked the start of a new decade and a period of rapid expansion driven by both organic growth and an ambitious acquisition strategy.
Between 2010 and 2019 Bimeda acquired medicinal, nutritional, diagnostics and vaccine businesses in Kenya, Brazil, the UK, US and France, as well as acquiring the veterinary medicine portfolio of Grupo Unipharm in Central America.
-
2011
Acquired
- Assia (Kenya)
- Mogivet (Brazil)
-
2012
Acquired
- Vetpharm Limited
(UK-based exports company)
- Vetpharm Limited
-
2013
Acquired
- Telsol Limited
(UK bolus technology)
- Telsol Limited
-
2014
Acquired
- Zootech (France)
-
2016
Acquired
- ArthroDynamic Technology Inc.
(Equine product specialist, USA) - NBVC (Nutrition, France)
- Iodolab (Diagnostics, France)
- ArthroDynamic Technology Inc.
-
2019
Acquired
- Veterinary Medicine Portfolio of Grupo Unipharm (Central America)
- Texas Vet Lab, Inc (USA)
(Vaccine manufacture and diagnostics)
-
2020
New Identity
- Texas Vet Lab Inc becomes Bimeda Biologicals
-
2021
Acquired
- AquaTactics Fish Health (USA)
-
2022
Acquired
- Afrivet Southern Africa Proprietary Limited (South Africa)
-
2023
Philanthropy
- UCD Bimeda Herd Health Hub & AgTechUCD Innovation Centre
Bimeda philanthropic donation
- UCD Bimeda Herd Health Hub & AgTechUCD Innovation Centre
During this period, Bimeda also joined Rainbow Laboratories Ltd, in establishing a raw material testing and innovation centre in Shijiazhuang, China.
2019 also saw the opening of Bimeda’s Global Innovation Centre in Dublin, Ireland, which leads our global innovation programme, in collaboration with five additional R&D laboratories in Minnesota (USA), Texas (USA), Monet Mor (Brazil), Shiazhuang (China) and Cambridge (Canada).

2020 and Beyond
 Bimeda China Shijiazhuang FacilityIn 2020, our state-of-the art sterile injectable manufacturing facility located in Shijiazhuang, China proudly commenced manufacturing operations. The facility is approved to supply quality veterinary pharmaceutical products to China, Australia, New Zealand, Kenya and Uganda.
Bimeda China Shijiazhuang FacilityIn 2020, our state-of-the art sterile injectable manufacturing facility located in Shijiazhuang, China proudly commenced manufacturing operations. The facility is approved to supply quality veterinary pharmaceutical products to China, Australia, New Zealand, Kenya and Uganda.
In 2021, Bimeda was pleased to enter the aquaculture sector, with the acquisition of AquaTactics Fish Health, in the USA. The same year, Bimeda established a new greenfield organisation in Denmark, Bimeda Nordic Aps. From early 2022 Bimeda Nordic commenced supply of our broad range of veterinary pharmaceuticals and animal health products to the Nordic region.
In 2022 Bimeda acquired Afrivet Limited in South Africa, resulting in Bimeda having the largest footprint of any animal health company in Africa.
In 2023 Bimeda made a substantial philanthropic donation to the UCD Foundation in Ireland. This donation was used to help fund the UCD Bimeda Herd Health Hub and AgTechUD Innovation Centre at UCD Lyons Farm in county Kildare, Ireland.
Today Bimeda employs over people worldwide and has a presence in over 80 countries. Our 9 manufacturing and 8 R&D facilities all operate to the highest regulatory standards, and our global supply chain and distribution network sees our products used to enhance animal health in all major food-producing regions.
Bimeda is an organisation which will continue to grow, continue to invest in innovation and continue to be committed to finding ever-better ways to serve our customer, as we continue strive for global excellence in animal health.
If you would like to join us on our exciting journey, check out our current vacancies.
Bimeda Vision, Mission and Values
Vision
To be the partner of choice for optimizing the wellbeing of the world’s animals.
Mission
As a family-owned company with over half a century’s experience in improving global animal health, we are passionate about providing our customers with efficient science-driven solutions to continuously optimise the wellbeing of animals.
Values
To succeed in our mission and vision, we are guided in our work by our Bimeda Values. Through our commitment to our Bimeda Values, we are able to continuously drive our business forward and surpass the expectations of our customers each and every day.
Our 5 Core Values
 Customer Focus
Customer Focus  Innovation
Innovation  Learning
Learning  Collaboration
Collaboration  Accountability
Accountability To learn more about Bimeda’s global presence, why not read our corporate brochure.
Careers
Think a career at Bimeda Canada could be right for you?
Learn about life at Bimeda Canada by downloading our ‘Why Bimeda?’ information pack.
Visit our careers website for all current career opportunities, or to submit your CV.
AODA INFORMATION
Should you wish to receive a copy of our AODA policy or Multi Year Accessibility Plan, please visit our accessibility page
About Bimeda Global
A Global Presence with Irish Roots
With half a century’s experience in the manufacture, distribution and marketing of veterinary pharmaceuticals and animal health products, Bimeda is a well-established global presence in the vet-pharma industry. Since our founding, in Ireland, in 1960, the company’s impressive growth has helped us achieve a truly global footprint that now stretches over 80 countries and across 6 continents.
9 manufacturing facilities
in 7 countries
More than
+ employees
worldwide
8 R & D and
7 Regulatory labs
Operations in
more than
80 countries
Global Manufacturing Capabilities
Bimeda has nine manufacturing facilities, across seven countries; all operating to the highest regulatory standards. We manufacture numerous dose forms, with the capacity for both sterile and non-sterile production.
Advanced Research & Development
While our customer-focused culture and an ethos of continuous improvement ensure customers’ current needs and expectations are routinely surpassed, our global R&D program is committed to anticipating the future needs of the animal health sector. Our continued investment in eight state-of-the-art laboratories across four continents is demonstrative of this commitment to serving the future needs of the global animal health market.
Vision
To be the partner of choice for optimizing the health and wellbeing of the world’s animals.
Mission
We are passionate about providing solutions to help animal lovers and animal health professionals optimize the health and wellbeing of animals around the world.
Values
To succeed in our mission and vision, we are guided in our work by our Bimeda Values. Through our commitment to our Bimeda Values, we are able to continuously drive our business forward and surpass the expectations of our customers each and every day.
Our 5 Core Values
 Customer Focus
Customer Focus  Innovation
Innovation  Learning
Learning  Collaboration
Collaboration  Accountability
Accountability More...

























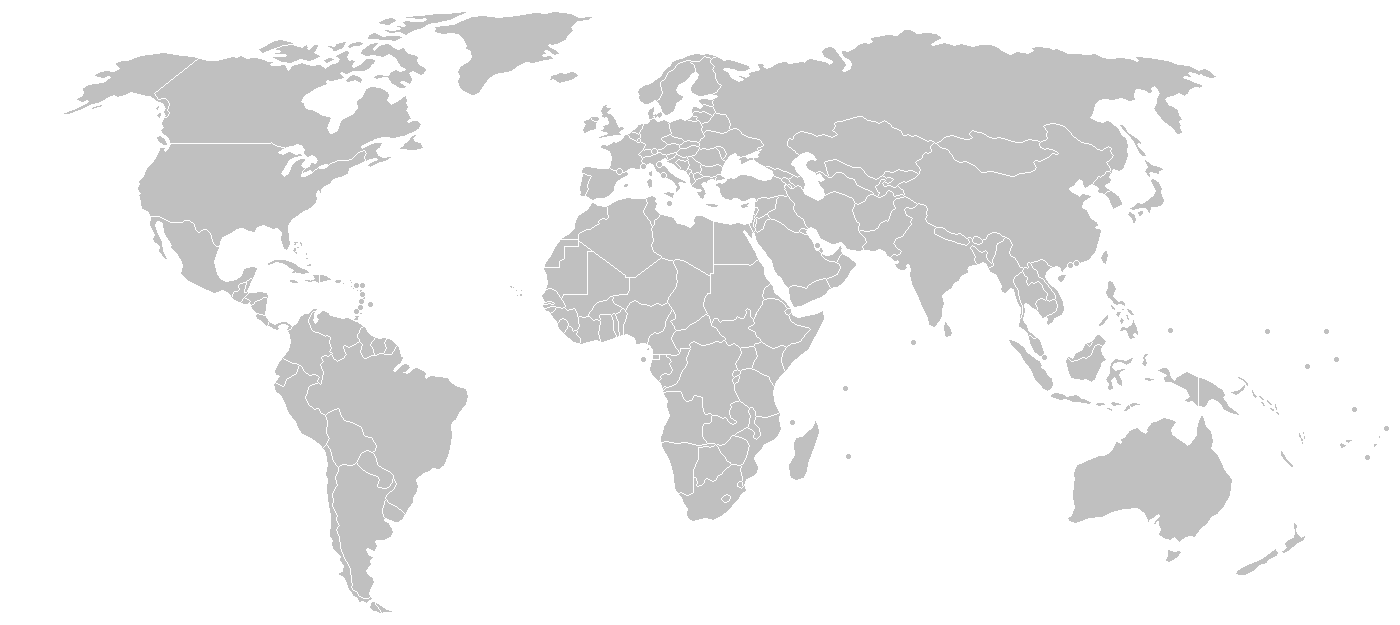
 Brazil
Brazil Canada
Canada China
China France
France Guatemala
Guatemala Ireland
Ireland Kenya
Kenya Mexico
Mexico UK
UK USA
USA Exports
Exports